Cytotoxicity assay of HER2-CAR T cells in agarose-coated wells. Access Additional Information About The Engineering Administration Of This Therapy.

Car T Cells Become Exhausted Upon Repeated Antigen Stimulation In Download Scientific Diagram
Reported a high-throughput multiparameter flow assay that combines CAR T-cell-mediated killing with concurrent evaluation of CAR T-cell transduction.

. Culture and collect Target cells and stain with CFSE 2. CAR T cells exhibit signifi- cant antigen specific cytotoxic activity against U87-EGFRvIII-Luc cells in vitro Fig. CAR-T cell cytotoxicity can be enhanced by this clustering and intracellular signaling can be amplified potentially exaggerating cytotoxicity values known as the.
Take A Look At Our Booklet Video. I used to perform the cytotoxicity assay. Best Pract Res Clin Haematol 312135146.
Ad World-class immunotherapy providing quality closed process CAR-T cell therapy platform. Chavez JC Locke FL 2018 CAR T cell therapy for B-cell lymphomas. This chapter describes the most common method for evaluating cytotoxicity of chimeric antigen receptor CAR T cells the xCELLigence real-time cell analysis RTCA platform Agilent.
Ad See The Mechanism Of Action For A 3L Large B-Cell Lymphoma CAR T Cell Therapy Option. Get The Patient Brochure And Learn About The TECARTUS Treatment Process. Cytotoxicity assay is an experimental method to evaluate the lead effecttoxicity measurement on the normal cell line.
Co-culture the Target cells with. A sensitive and reproducible cytotoxicity assay that collectively reflects these functions is an essential requirement for translation of these cellular therapeutic agents. 22Remove the cell plate from the incubator and aspirate off growth medium.
An improved flow cytometric assay for the determination of. 23Add treatments and controls to appropriate wells of the 96-well plate. In this paper we have developed a.
Results are comparable to those previ- ously published. Current assays lack the sensitivity and. Therapy studies whist miniaturizing the assessment of CAR-T therapeutic strategies thus decreasing assay costs and time to results.
The analysis mainly contains chromium release cytotoxicity. Ad Visit The Official Patient Site To Learn More And Read Medication Guide. Creative Biolabs offers CellRapeutics cytotoxicity test for CAR-T cells.
Ad Find Out If CAR T Cell Immunotherapy Is Right For You. CAR engrafted T cell or NK cell may be toxic to normal cells which also express some tumor associated antigens on. The chromium 51 Cr-release assay 51 Cr assay the luciferase.
T cells were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies mAbs in the presence of IL-2. IncuCyte-based cell therapy assays adherent. CAR-T cell induced cytotoxicity Untransduced PBMC.
Creative Biolabs offers tumor lysis analysis during CAR-T cell evaluation in animal models for toxicity and safety assessment. The short protocol is like that first I labeled the target cell with CFSE Co-culture with CAR-t and then next day stained with PI and analyzed by FACS. In vitro CAR-T and cell therapy testing assays to evaluate on-target efficacy and off-target or on-targetoff-tumor activity of cellular therapies.
Great predictability and a streamlined approach in treatment of various types of cancer. Ad Get The CAR T Discussion Guide - For Relapsed Large B-Cell Lymphoma. In other words the designed or identified lead should be tested.
The y-axis is showing the fold change in the number of GFP positive HEK293 cells in comparison to 0-hour time point. T cells exhibit cytotoxicity against U87-EGFRvIII glioma cells. Potential over-activation or off-target effects are part of early development and lead.
We specifically focus on four of the most commonly used assays to investigate cell-mediated cytotoxicity. Cancer Support Community Provides Support For Newly Diagnosed People And Their Loved Ones. Get Tips For Discussing CAR T With Your Oncologist Locate Nearest Treatment Hospital.
Neither target cell viability nor CAR T-cell activation changes when CD19- target cells are co-cultured with CAR T-cells. The Long Evolution Of CAR-T Assays. CAR T Cytotoxicity Assay Procedure Example This protocol was used for measuring LDH cytotoxicity of CAR-modified T cell and Daudi target cell in RPMI Medium Modified media.
Martinez et al. Add the CAR T cells at different ET ratios 4. July 22 2019 The evolution of CAR-T cell study has been a long process says Preet Chaudhary.
Reported a high-throughput multiparameter flow assay that combines CAR T cellmediated killing with concurrent evaluation of CAR T-cell transduction and activation. Get Tips For Discussing CAR T With Your Oncologist Locate Nearest Treatment Hospital. Ad Get The CAR T Discussion Guide - For Relapsed Large B-Cell Lymphoma.
A simple and sensitive method to quantitatively measure the cytolytic effect of tumor-specific T killer cells is highly desirable for basic and clinical studies. But eventually CAR T cells or TCRs will need to be tested in vitro with primary human tissues. Seed the Target cells in the wells of 96-well microplate 3.

Real Time Potency Assay For Car T Cell Killing Of Cancer Cells
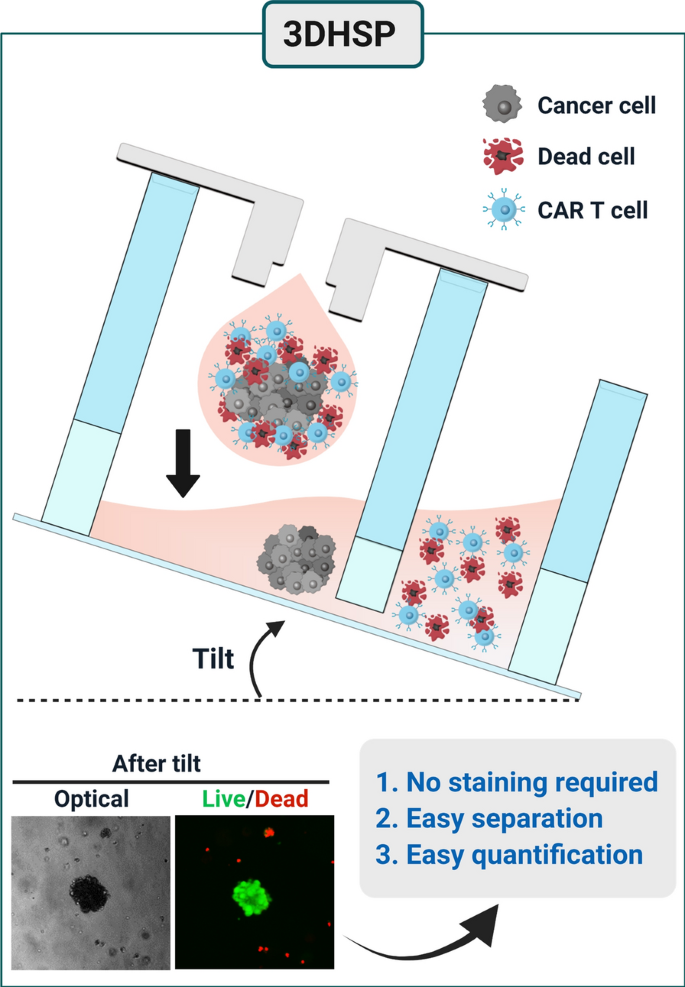
3d Hanging Spheroid Plate For High Throughput Car T Cell Cytotoxicity Assay Journal Of Nanobiotechnology Full Text

Jci Perforin Deficient Car T Cells Recapitulate Late Onset Inflammatory Toxicities Observed In Patients
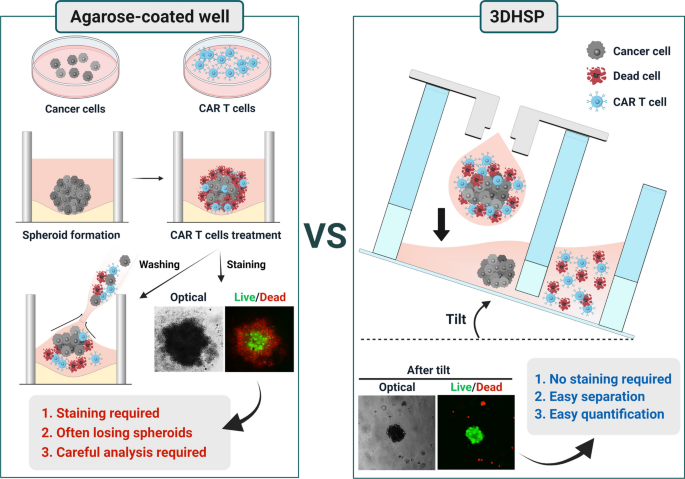
3d Hanging Spheroid Plate For High Throughput Car T Cell Cytotoxicity Assay Journal Of Nanobiotechnology Full Text
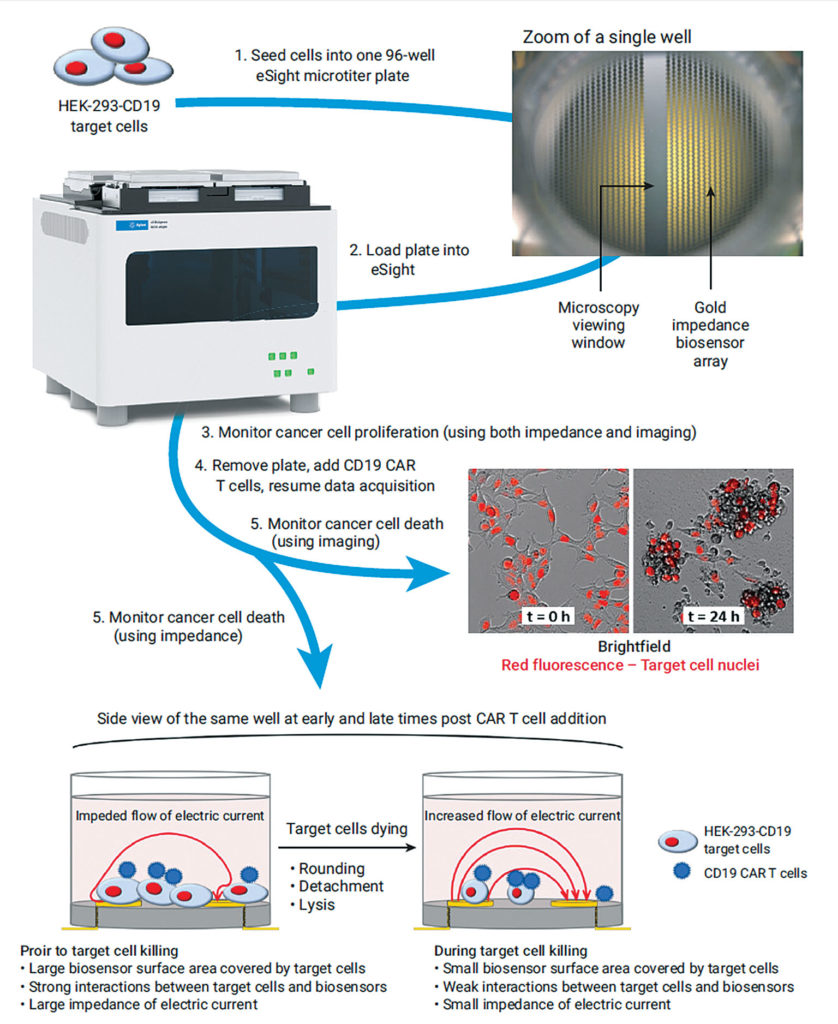
Real Time Potency Assay For Car T Cell Killing Of Cancer Cells

In Vitro Evaluation Of Car T Cells In Patient Derived Glioblastoma Models Star Protocols

Real Time Potency Assay For Car T Cell Killing Of Cancer Cells
0 comments
Post a Comment